A) A
B) B
C) C
D) D
E) E
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains hydroxyl groups as its predominant functional group. Which of the following statements is true concerning this compound?
A) It lacks an asymmetric carbon, and it is probably a fat or lipid.
B) It should dissolve in water.
C) It should dissolve in a nonpolar solvent.
D) It won't form hydrogen bonds with water.
E) It is hydrophobic.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They are hydrophilic.
D) They exhibit considerable molecular complexity and diversity.
E) They are lighter than water.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes the carbon atoms present in a seed-eating bird?
A) Inorganic carbon atoms in the seeds were incorporated into organic molecules by the bird.
B) The carbon atoms ultimately came from the soil.
C) The carbon atoms are ultimately derived from coal.
D) The carbon atoms ultimately came from carbon dioxide incorporated into sugars through photosynthesis.
E) The carbon atoms ultimately came from simple organic compounds that formed abiotically from inorganic carbon, hydrogen, oxygen, and nitrogen atoms.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
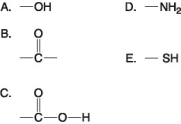 -Which of the groups above is a carboxyl functional group?
-Which of the groups above is a carboxyl functional group?
A) A
B) B
C) C
D) D
E) E
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many structural isomers are possible for a substance having the molecular formula C₄H₁₀?
A) 1
B) 2
C) 4
D) 3
E) 11
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acids are acids because they always possess which functional group?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
E) hydroxyl
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that
A) have identical chemical formulas but differ in the branching of their carbon skeletons.
B) are mirror images of one another.
C) exist in either linear chain or ring forms.
D) differ in the location of their double bonds.
E) differ in the arrangement of atoms around their double bonds.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes the carbon atoms present in a seed-eating bird?
A) They were incorporated into organic molecules by plants.
B) They were processed into sugars through photosynthesis.
C) They are ultimately derived from carbon dioxide.
D) They were incorporated into organic molecules by plants, and they are ultimately derived from carbon dioxide.
E) They were incorporated into organic molecules by plants, they were processed into sugars through photosynthesis, and they are ultimately derived from carbon dioxide.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Stanley Miller's 1953 experiments proved that
A) life arose on Earth from simple inorganic molecules.
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth.
C) life arose on Earth from simple organic molecules, with energy from lightning and volcanoes.
D) the conditions on early Earth were conducive to the origin of life.
E) the conditions on early Earth were conducive to the abiotic synthesis of organic molecules.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemist wishes to make an organic molecule less acidic. Which of the following functional groups should be added to the molecule in order to do so?
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
E) phosphate
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following hydrocarbons has a double bond in its carbon skeleton?
A) C₃H₈
B) C₂H₆
C) CH₄
D) C₂H₄
E) C₂H₂
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
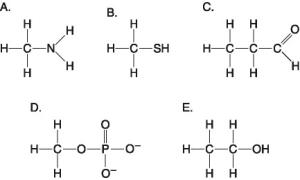 -Which molecule shown above is a thiol?
-Which molecule shown above is a thiol?
A) A
B) B
C) C
D) D
E) E
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organic molecules with only hydrogens and five carbon atoms can have different structures in all of the following ways except
A) by branching of the carbon skeleton.
B) by varying the number of double bonds between carbon atoms.
C) by varying the position of double bonds between carbon atoms.
D) by forming a ring.
E) by forming enantiomers.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complexity and variety of organic molecules is due to
A) the chemical versatility of carbon atoms.
B) the variety of rare elements in organic molecules.
C) the fact that they can be synthesized only in living organisms.
D) their interaction with water.
E) their tremendously large sizes.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 -Three or four of the following illustrations depict different structural isomers of the organic compound with molecular formula C₆H₁₄. For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted. Which one, if any, is NOT a structural isomer of this compound?
-Three or four of the following illustrations depict different structural isomers of the organic compound with molecular formula C₆H₁₄. For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted. Which one, if any, is NOT a structural isomer of this compound?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) Each of the illustrations in the other answer choices depicts a structural isomer of the compound with molecular formula C₆H₁₄.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which functional group is not present in this molecule?
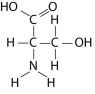
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon skeleton is covalently bonded to both an amino group and a carboxyl group. When placed in water it
A) would function only as an acid because of the carboxyl group.
B) would function only as a base because of the amino group.
C) would function as neither an acid nor a base.
D) would function as both an acid and a base.
E) is impossible to determine how it would function.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 -Which pair of molecules shown below are not enantiomers of a single molecule?
-Which pair of molecules shown below are not enantiomers of a single molecule?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
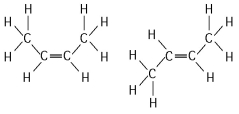
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms, but with one or more double bonds, will
A) be more flexible in structure.
B) be more constrained in structure.
C) be more polar.
D) have more hydrogen atoms.
E) have fewer structurally distinct isomers.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 71
Related Exams